Mitigating the risk of harm during the transition from controlled to assisted mechanical ventilation
Monitoring the strength of the patient's breathing effort, titrating the sedation, and selecting the correct mode of ventilation is vital when transitioning from controlled to assisted ventilation.
Here to guide us towards patient liberation, using monitoring techniques with personalized lung- and diaphragm-protective ventilation is Dr Irene Telias, one of the medical world’s foremost experts on respiratory physiology.
Key learnings in this article.
- Avoiding potentially injurious efforts
- Addressing over-sedation, dyssynchrony, diaphragm atrophy, excessively high or low breathing efforts, over-assistance and more
- Monitoring techniques and their benefits and limitations
- Ventilation modes of benefit during the transition
Life-saving, but potentially injurious
Mechanical ventilation is an intervention that is frequently used in acutely ill adult patients requiring either respiratory support or airway protection. The ventilator facilitates gas exchange while other treatments are deployed to improve the patient’s clinical condition. Despite being a life-saving measure, mechanical ventilation can be potentially injurious for patients and therefore careful management of the ventilator settings and drugs used during mechanical ventilation are key to prevent harm.
Assessing the transition from fully controlled to assisted ventilation
One of the key issues with mechanical ventilation is the transition from fully controlled to assisted mechanical ventilation, which must be achieved as soon as possible to improve the patient’s outcomes. However, this transition must be accomplished in a manner that avoids worsening the patient’s respiratory problems, thereby forcing the ICU team to resume fully controlled ventilation.
To distinguish between breaths with no active respiratory muscles (fully controlled ventilation) and breaths with active muscles (assisted ventilation), we need to monitor the patient’s breathing effort. Dr Irene Telias has been a fellow in Toronto from 2015 to 2017, initially as a clinical fellow, and then as a combined research and clinical fellow. She is now in Toronto pursuing a PhD focused on applied respiratory physiology at the University of Toronto.
Irene is one of the medical world’s foremost experts on respiratory physiology, more specifically respiratory effort during mechanical ventilation in intensive care (ICU) and its influence on ventilation-induced lung injury and diaphragmatic dysfunction. When assessing the transition from fully controlled to assisted mechanical ventilation, Irene always uses monitoring techniques to titrate mechanical ventilation and sedation to avoid potentially injurious effort. “An intermediate range of inspiratory effort is associated with better outcomes for patients and might be a reasonable target for most patients,” Irene says.
An intermediate range of inspiratory effort is associated with better outcomes for patients and might be a reasonable target for most patients.

Evaluating the transition’s challenges
The transition from full sedation and controlled ventilation to spontaneous breathing is always very challenging, she says. ”Why? Firstly, the patient is only partially conscious of what's happening, and usually experiencing sharp discomfort with a tube down their throat,” Irene says.
Secondly, under sedation there are many stimuli telling the brain to breathe strongly. Patients are often still very sick. For example, systemic inflammation due to an unresolved or new infection is a strong direct stimulus for the patient to breathe. “Because the patient is breathing and the ventilator is providing support at the same time during this critical period, matching the timing of the patient’s own breathing pattern and that of the ventilator's insufflation and exhalation is critical. If there is a lack of synchrony between those events, patient-ventilator dyssynchrony occurs, a phenomenon that is associated with increased patient mortality ,” Irene says.
Irene continues: “This patient-ventilator dyssynchrony can be very uncomfortable for the patient, and potentially injurious for the lungs and the patient's main respiratory muscle, the diaphragm.” How are these challenges addressed? How can clinicians adapt or tailor mechanical ventilation to avoid harm to the patient in the process of transitioning from fully controlled ventilation to assisted ventilation?
When assessing the transition from fully controlled to assisted mechanical ventilation, Irene always uses monitoring techniques to titrate mechanical ventilation and sedation to avoid potentially injurious effort.
Personalizing mechanical ventilation and sedation
According to Irene, studies have shown that patients with an intermediate range of inspiratory efforts – not excessive, and not too shallow – have better ICU outcomes, including lower mortality rates. However, one size does not fit all and personalizing the treatment is necessary. “There are several important ways to personalize patient care so that the chances of an intermediate range of inspiratory efforts are increased,” Irene says. “First, the mode of mechanical ventilation we use is important, as is how much support the ventilator offers, and how we adapt the breathing pattern provided by the ventilator according to the patient's breathing pattern so that the patient is breathing in synchrony with the ventilator while the ventilator is providing the support. That's one element of this lung- and diaphragm-protective ventilation strategy - managing the ventilator setting.”
The second part of the patient personalization process is the use of sedation to modulate the respiratory drive. The sedative agents that are most often used to do this are propofol and benzodiazepines. “However, doctors have to very carefully titrate these drugs,” Irene says, “because if you use excessive amounts of these drugs you might generate adverse effects. For instance, if the patient is on very high doses of sedative agents for a long time, they might suffer from respiratory muscle and peripheral muscle atrophy because they haven’t moved for several days.” Factors other than management of the ventilator settings and sedation are also important, such as understanding and treating the reason for excessively high or low breathing efforts. For example, patients are often uncomfortable or anxious and these factors must be addressed.
Monitoring techniques that help facilitate the transition
How do we monitor the strength of the patient's breathing effort and, therefore, target an intermediate range of effort facilitating the transition from fully controlled to assisted ventilation? There are several monitoring techniques that can help the transition.
Esophageal pressure (Pes) for example, is the gold standard of measuring a patient’s inspiratory effort and the risk of harm. This measurement technique involves passing a Pes catheter with a balloon tip into the midesophagus to record the local pressures as a surrogate for the intrathoracic pressure. When we breathe, we suck air inside our lungs by decreasing intrathoracic pressure. Pes measures the change in intrathoracic pressure generated by the respiratory muscles.
According to Irene, there are two other techniques that are simpler and less invasive because neither of the two, the Pocc and the P0.1, require the insertion of a catheter.
“These are measured with the ventilator, so we call them non-invasive monitoring techniques,” Irene says. “They both rely on the same principle; that we generate what we call an end-exp iratory hold. That's like a breathhold while the patient breathes in. When the patient breathes in against a closed airway, any change in airway pressure is proportional to the change in intrathoracic pressure. It's a trick that we use to mimic what we would see if we were to have inserted an esophageal catheter. We're able to measure changes in intrathoracic pressure without having to put anything in the thorax. These techniques are used to measure the patient's respiratory drive to check if the efforts are too high or too low. Once you have a more detailed assessment of the patient's drive and effort, you may need to go with a little more invasive technique, such as Pes, but these two techniques, Pocc and P0.1, are screening techniques that can be used in all ventilated patients because you don't need to insert anything.”
Another available technique to monitor patient's respiratory drive and effort is the electrical activity of the diaphragm (Edi). Like Pes, it requires the insertion of a naso- or orogastric catheter. However, Edi catheters always contain a feeding tube as well, which is needed in almost all situations. An Edi catheter is connected to, and the signal is processed by the ventilator. The Edi signal is directly displayed on the ventilator's screen, providing information about the magnitude and timing of the patient's drive and breathing effort. It therefore allows clinicians to modify ventilator settings and drugs to ensure that the patient exerts a safe amount of effort and there is a better patient-ventilator synchrony.
The good thing about this mode is that because it is proportionate to the patient's drive and effort, it is very unlikely that the ventilator will provide too much support – what we call over-assistance.
Modes of ventilation that are of potential benefit during transition
Selecting the best ventilator mode and settings for each patient is one of the most important interventions to achieve a lung- and diaphragm-protective ventilator strategy. NAVA is an important tool for many patients. It is a proportional ventilatory mode that uses the Edi to offer ventilatory assistance in proportion to patient drive and effort.
“The good thing about this mode is that because it is proportionate to the patient's drive and effort, it is very unlikely that the ventilator will provide too much support – what we call over-assistance,” Irene says.
Other modes, such as pressure support, can overassist or under-assist the patient. Pressure support is the mode that is most frequently used during the transition from fully controlled to assisted mechanical ventilation.
“Pressure support provides a fixed amount of support for each breathing effort,” Irene says. “If the ventilator provides a fixed amount of support for each breathing effort, the patient’s breathing effort is likely to decrease when the ventilator provides support. If the support is too much for the patient, they will take a small inhalation that initiates the breath, but then during the whole breath, the patient is passive. When the patient falls asleep in this situation, sometimes they even become apneic, which means they don't take a breath for several
seconds. The patient may wake up gasping for breath, something that obviously disrupts sleep. That's called apnea during pressure support. Sleep disruption is a major problem in ICU. You can imagine it's very difficult to have a proper restorative sleep in ICU. We think that sleep has a very important physiological function, specifically for healing, so we prefer to use methods that will encourage better sleep quality and quantity.”
According to Irene, proportional modes, such as NAVA and proportional assisted ventilation, have the potential to avoid this phenomenon and might result in a better sleep quality and quantity in ICU.
“The amount of support is proportional to the patient’s efforts,” Irene says, “so this ensures the patient continues to exert some degree of breathing effort, and they rarely become apneic, decreasing the risk of sleep disruption, and diaphragmatic atrophy . The main potential benefits of this mode are that you improve patient ventilator synchrony, avoid over-assistance and respiratory muscle atrophy, and endure less sleep disruption. Most patients might benefit from NAVA, except those who have excessively high respiratory drive and effort due to an abnormal brain function and will not respond to adjustments in the ventilator settings or sedation. In these circumstances, proportional modes, including NAVA, sometimes provide excessive assistance which might exacerbate patient lung injury.”
In conclusion, despite the transition between fully controlled to assisted mechanical ventilation being extremely challenging, current available monitoring techniques, together with the safe implementation of proportional modes of ventilation, can help achieve an effective and personalized lung- and diaphragm-protective ventilation which will ultimately move towards patient liberation.
Monitoring techniques
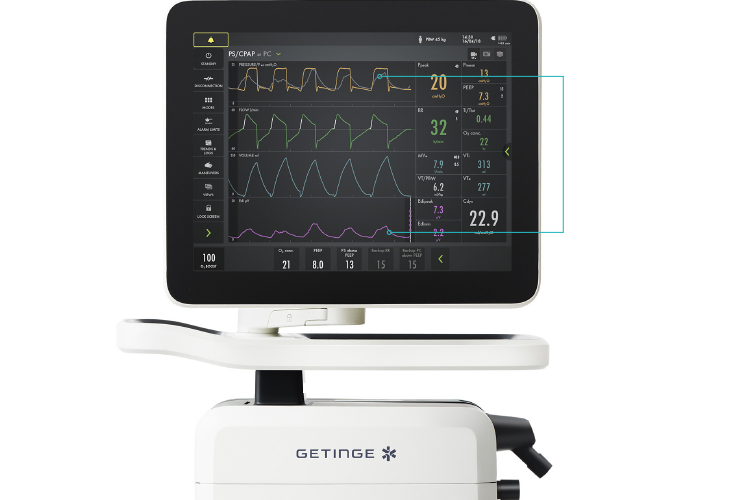
The electrical activity of the diaphragm (pink) can help clinicians to modify ventilator settings and drugs to ensure that the patient exerts a safe amount of effort and that there is a better patient-ventilator synchrony (grey overlay at pressure curve, yellow).
