Choosing the right chemistry for surgical instrument cleaning efficiency
How do you know which chemistry is best for cleaning your surgical instruments? Or which chemistry will work best in which step of the process? You’ll want to find the most cost-efficient product with the best performance for your processes.
Water matters: Understanding the impact of water quality on cleaning chemistries in sterile processing
Ask yourself the right questions before you decide. Can you use a universal detergent? Or do you need separate ones for specific processes? How is your facility’s water quality? Do you need to counteract contaminants in the water or use a different source? Let’s take a closer look at cleaning chemistries and break down their ingredients and the role each plays in sterile processing.
The first thing we need to look at is your facility’s water. As more companies work toward sustainability, more chemistries are available in concentrated form to reduce packaging. They are then mixed with water in the sterile processing department (SPD). The type of water used and how it interacts with both chemistries and surgical instruments makes a difference.
Municipal water may be “hard water,” meaning it contains metals and impurities that can damage delicate surgical instruments. Some facilities use reverse osmosis or deionized water, instead of tap water. Deionized water removes those impurities, but also removes the chlorine used to kill microorganisms. Reverse osmosis filtering removes minerals, metals, and microorganisms.
For facilities using municipal water, many detergents contain chelating or sequestering agents to counteract hard water and extend the life of your instruments. They soften water by binding with mineral ions, inactivating them by changing their charge, and keeping them surrounded in solution. Without this, the minerals can create deposits on the surgical instruments that can hide microbes and prevent them from being killed during sterilization. For facilities using deionized water, some chemistries contain preservatives, which have antimicrobial properties.
The role of enzymes and pH: Key elements in effective sterile processing detergents
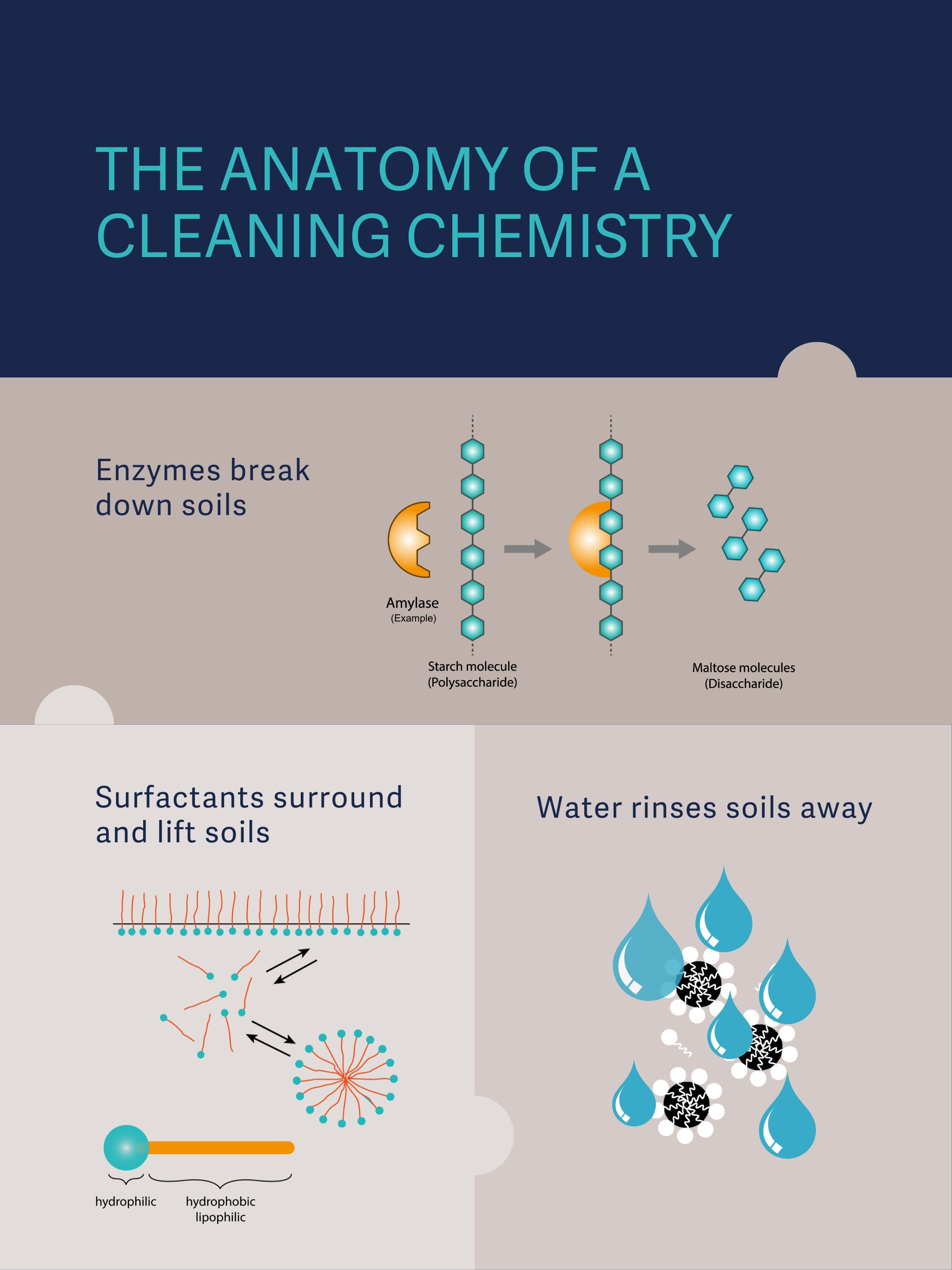
In addition to the water used to dilute them, two of the most important features of cleaning chemistries are enzymes and pH. In sterile processing, most detergents contain one or more enzymes to break down the organic soils deposited on instruments during surgery. Different enzymes attack different soils. Many detergents contain multiple types of enzymes to handle all possible surgical soils and make the solutions more universal.
The most common enzymes included are proteases, lipases, and amylases. Quadruple enzyme detergents also contain cellulases. Protease enzymes break down proteins such as blood and tissue. Lipases affect fats and oils. Amylases attack starches, carbohydrates, and sugars. Cellulases break down cellulose and have shown some efficacy in attacking bacterial biofilms.[1]
Enzymatic cleaners are not recommended for ophthalmic instruments, however, as they have been linked to Toxic Anterior Segment Syndrome (TASS).
Why are enzymes important? They not only make cleaning happen faster and more efficiently, they are more sustainable and safer for SPD staff than harsh chemicals. Enzymes are catalysts. They speed up the reactions that break down soils and allow those reactions to use less energy. They make it easier for surfactants to lift and remove the soils from instruments, including inside lumens and robotic instruments.
Enzymes are naturally occurring and completely biodegradable. Their low toxicity profile means they are safe for SPD technicians to handle and safe to use on a wide variety of materials.[2] To be effective in detergents requires only a low concentration, reducing packaging and storage needs. Because enzymes degrade over time, stabilizers are added to lengthen their shelf life.
Intermolecular dynamics: The role of surfactants
Surfactants work hand in hand with enzymes to remove soils. They are added to detergents to break the surface tension of water, emulsify oils, and lift soils from the surfaces of the instruments. They are made up of two parts: a water-loving (hydrophilic) head and a water-hating (hydrophobic) tail that is attracted to soils.
The surfactants first disrupt the surface tension of the liquid, then once enough of them are present, their tails surround the soils, lifting them from the surface of the instrument, and then they form a group (called a micelle) with their heads on the outside and their tails on the inside. Once the soils are encased in a micelle, they are easily rinsed off (surfactants are also used in rinsing chemistries to improve sheeting action). Some surfactants have antimicrobial properties, as well.
While surfactants can have positive or negative charges on their hydrophilic ends, those used for sterile processing are often nonionic, which means they have no charge. Nonionic surfactants are particularly good at removing soils and are low-foaming. Reducing foam levels means greater water clarity.
Water clarity is important for technician safety when manually washing sharp instruments or reaching into an ultrasonic cleaner. Too much foam also can clog the spray arms in a washer-disinfector, reducing water pressure and the machine’s cleaning efficacy, and clog lumen instruments being flushed in automated washers. Reducing the foam can save you from repeated machine errors and service calls.
pH impact: Cleaning chemistry's influence on instrument materials in sterile processing
Next, we’ll look at how pH affects your cleaning chemistry. A solution’s pH determines how it interacts with the materials making up the instrument. Too much acidity or alkalinity can cause pitting, staining, or corrosion of metal instruments or erosion of plastics and rubbers. Many cleaning chemistries have a neutral pH to make them compatible with the widest range of instruments, including flexible endoscopes. Enzymatic detergents have a neutral pH.
Alkaline detergents are best for use in hard water but may not be compatible with soft metals like aluminum. They are most often used in automated washers, where an acidic neutralizing rinse can be applied after the wash cycle. Mildly alkaline formulas, however, can be used with softer metals and may not need a neutralizing rinse. The alkalinity dissolves protein and fat residues.
Acidic chemistries may be used in rinses to brighten, destain and repassivate stainless steel instruments (coat them to make their surfaces resistant to rust and corrosion), and to improve sheeting action and drying to prevent water spots and stains. Acidic blends, often made from phosphoric acid and nitric acid, are also used in equipment maintenance. The acids remove rust stains, scale, discoloration, and mineral deposits from equipment chambers and reduce corrosion.
Corrosion inhibitors are used in many sterile processing chemistries to protect metal instruments. They form a light film on metal surfaces that prevents corrosion and extends the life of the instruments. Preservatives are added to stabilize the solution and prevent the growth of bacteria and molds.
Sustainable formulations: Prolonging instrument life, and minimizing environmental impact
One final consideration is the sustainability of the product, as many companies and facilities are making this a priority. With the right ingredients, modern chemistries protect and extend the life of your surgical instruments so you can replace them less frequently. Their formulations also protect patients and staff from inhalants, bacteria, and other microbes.
As mentioned earlier, many chemistries are more concentrated than in the past, as well. Some concentrations can achieve up to 10x the number of cycles per bottle compared to equivalent products. This adds up to less time spent on ordering, less packaging, and lower shipping costs.
Is it time to update your chemistries? Determine what type and number of instruments your SPD is processing. Take a look at the machines you are using and the types of chemistries they require. Then compare the ingredients in your current chemistries to newer ones to make sure you are using the most cost-efficient product with the best performance.
