The importance of reprocessing your instruments

Effectively reprocessing your Reusable Medical devices is not only critical in preventing the spread of healthcare-associated infections, it is a requirement.[1]
Every day healthcare providers reprocess a variety of instruments at different times, temperatures and conditions. The one thing you want is assurance that your goods have been thoroughly cleaned and sterilized in accordance with your local standards and facility requirements. As your full-service partner, we support you through the entire clinical pathway, so that you can continue to provide excellent care, without compromise.
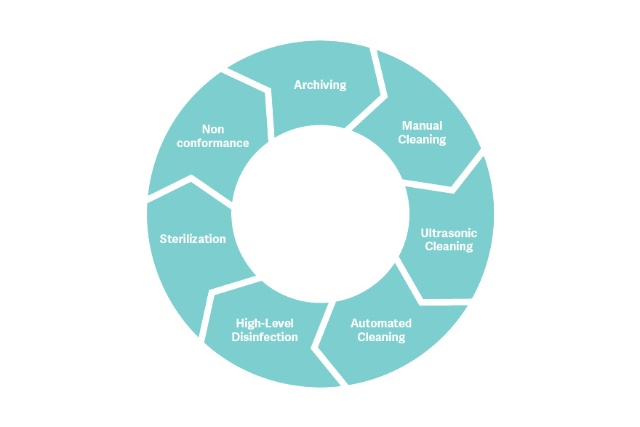
The healthcare reprocessing workflow

The Meditrax range offers traceability systems aligned to AS/NZS 4187 Standards that are easy for staff to use.
The Getinge Assured range delivers independent verification and documentation that a process has been performed.

Getinge Pack Sterilization pouches and rolls are printed with water based, non-toxic ink to produce Type 1 chemical process indicators in compliance with ISO 11140-1 standard.

What is MEDITRAX®?
MEDITRAX® is our manual instrument tracking system that allows you to record every step in the sterile supply workflow.
- An easy-to-use traceability system to help comply with AS/NZS 4187:2014 Standards.
- Record & track data on single instruments and instrument trays.
- Components available for the entire sterile supply flow.
- Cost-effective alternative to electronic traceability solutions.
Why do I need MEDITRAX®?
According to Australian & New Zealand Standards AS/NZS 4187:2014 health service organisations are required to carry out effective and safe reprocessing of RMDs. This is essential to protect patients and staff.
The purpose of traceability according to AS/NZS 4187:2014:
To have documented evidence of all items that have been through a sterilizing processes assists in recall of an item or load if necessary and recording of load contents for statistical data for load volumes.

What does that mean for me?
Traceability – can you trace your RMD right from decontamination right through to the patient?
Accountability – can you identify the individual responsible through the different stages of the sterile workflow?
Quality Assurance – can you use your traceability system as an integral component of your quality assurance system that enables corrective actions and staff training within the sterile supply department?
Defence against litigation – can you rely on your traceability system as proof that RMDs used on patients have undergone and passed all necessary processes?
What do the standards actually say?
2.4.3.2 Traceability records shall require at a minimum, the identification of the following for each RMD:
(a) Disinfection Process Records
i) Type of RMD
ii) Unique identification number of the RMD
iii) Date of cleaning & identification of the person responsible.
v) Identification of the Machine/ equipment
vi) Disinfecting process cycle number and date
vii) Documented evidence of attainment of process parameters, e.g. process record/printout and test/control results
viii) Identification of the person responsible for the release of the RMD.
(b) Sterilization Process Records
i) Date of sterilization and sterilizing process cycle number.
ii) Identification of the sterilizer e.g Machine number.
iii) Identification of the RMD (e.g. RMD name or name of a set of RMDs) and the number of these items within the load.
iv) Identification of the person responsible for loading the RMDs into the sterilizer.
v) Other records, e.g. performance/monitor test results
vi) Documented evidence of attainment of process parameters, e.g. process record/printout
vii) Identification of the person responsible for the release of the RMD/load.